Anna P Ainslie, John Robert Davis, John J Williamson, Ana Ferreira, Alejandro Torres-Sánchez, Andreas Hoppe, Federica Mangione, Matthew B Smith, Enrique Martin-Blanco, Guillaume Salbreux, Nicolas Tapon. Current Biology (2022).
Abstract
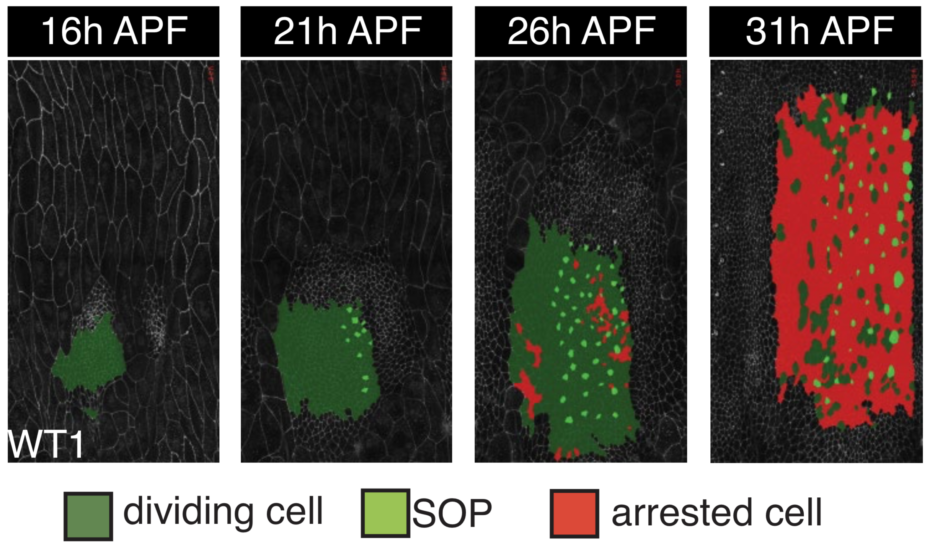
During development, multicellular organisms undergo stereotypical patterns of tissue growth in space and time. How developmental growth is orchestrated remains unclear, largely due to the difficulty of observing and quantitating this process in a living organism. Drosophila histoblast nests are small clusters of progenitor epithelial cells that undergo extensive growth to give rise to the adult abdominal epidermis and are amenable to live imaging. Our quantitative analysis of histoblast proliferation and tissue mechanics reveals that tissue growth is driven by cell divisions initiated through basal extracellular matrix degradation by matrix metalloproteases secreted by the neighboring larval epidermal cells. Laser ablations and computational simulations show that tissue mechanical tension does not decrease as the histoblasts fill the abdominal epidermal surface. During tissue growth, the histoblasts display oscillatory cell division rates until growth termination occurs through the rapid emergence of G0/G1 arrested cells, rather than a gradual increase in cell-cycle time as observed in other systems such as the Drosophila wing and mouse postnatal epidermis. Different developing tissues can therefore achieve their final size using distinct growth termination strategies. Thus, adult abdominal epidermal development is characterized by changes in the tissue microenvironment and a rapid exit from the cell cycle.